Nitrate Preconcentration
Introduction
This document is a copy and paste from three renditions of the original Word document created by Joseph Erbland. Joseph's is first, followed by Lei Geng's, and lastly Sydney Clark's from HEEL
Recent publications using the resin
Consider using HCl instead of NaCl because NaCl has nitrate in it while it is possible to purchase HCl without nitrate. Here are two recent publications that may help implement this strategy:
- Kawashima H, Yoshida O, Suto N. (2020). Ion-exchange resin and denitrification pretreatment for determining δ15N-NH4+, δ15N-NO3−, and δ18O-NO3− values. Rapid Communications in Mass Spectrometry 35. doi:10.1002/rcm.9027
- Marzaioli F, Di Rienzo B, Stellato L, Di Fusco E, Rubino M, D'Onofrio A, Terrasi F (2021). Characterization of the analytical performance of δ15N and δ18O measurements by the silver nitrate method in the framework of nitrate source apportioning. Rapid Communications in Mass Spectrometry 35. doi:10.1002/rcm.9009
Joseph Erbland's Protocol (c 2010)
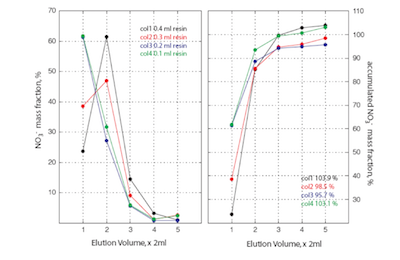
In order to guarantee a good repeatability of the isotopic measurements with the IRMS, a minimum of 100 nmol of nitrate is needed. Since nitrate ice core concentrations fluctuate between 10 to 800 ppbw, we need to process between 7 to 620 ml of water sample which overpass in most case the maximum volume allowed in the denitrifying bacteria vials (10 ml of sample max). It is therefore necessary to preconcentrate most of ice core samples for nitrate isotope analysis. This is achieved by passing the necessary water sample through an anion exchange resin which traps the nitrate and all other anion species. The nitrate and others species are then eluted with a 1M NaCl solution. Trapping and extraction are quantitative (Figure 1), limiting any possible isotopic fractionations associated with the extraction technique.


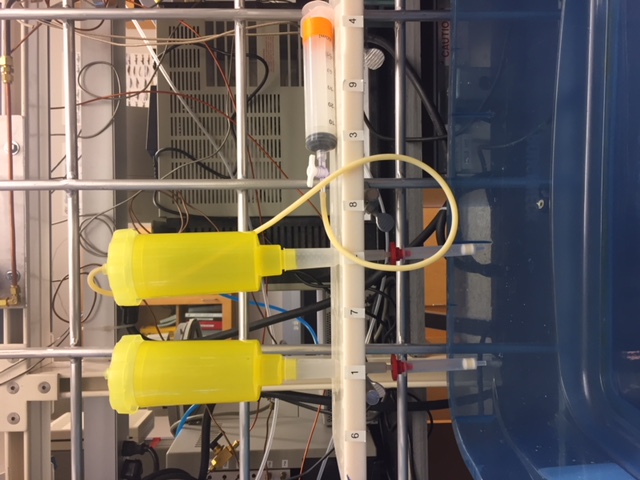
- Nitrate concentration in water sample need to be determined accurately to fix the volume of sample necessary to achieve 200-300 nmol of nitrate (absolutely necessary for at least duplicates).
- Prepare a small vial (accuvettes) by mixing roughly 50% v/v of resin (AG I-X8, 200-400 mesh, Chloride form, BIO-RAD) and 50% v/v of NaCl 1M
- Shake vigorously the vial to homogenise the resin solution
- Prepare the resin column (polyprep column, BIO-RAD) by pipetting 0.6 ml of this solution and transferring to the column, verify that you have effectively ~ 0.3 ml of resin
- Rinse the resin with 3 x 3ml of NaCl (1M) and after 3 x 3ml of Millipore water
- Pass your water sample through the resin. The excess water can flow to a sink
- Place your clan vial (accuvettes) below your column
- Elute with 5 x 2 ml 1M NaCl, close the vials, label
- Rinse with 3 ml of Millipore water the resin. The same columns are ready to be used for another sample.
- If the resin turns grey or black, or if you suspect any contamination, change the resin as described before. Depending on the purity of the sample, the same resin can be used many times.
- Note: Record the sample volume (or mass) processed for each sample and its respective concentration, this will avoid us to re measure the nitrate concentration in a NaCl matrix and will allow us to calculate the volume needed to be injected in the bacteria solution (again the target is 100 nmol for one measure)
Lei Geng's protocol (c 2015)
Concentration of Nitrate in Summit Ice Core
- Ice sample preparation
- Refer to cut plan, cut and decontaminate sample: sample size ≥ 500ml (equivalent water size, based on ice density to calculate minimum length of ice).
- Record sample information: Tube number, depth in core, sample number, sample size, and date.
- Save decontaminated samples in clean covered cups; measure and record the volume of melt water: weigh the dry pre-cleaned cups before and after adding samples, then using the mass of ice divide by water density (1.00g/ml).
- After melting, take 5 ml of melt water to measure nitrate concentration by IC, calculate the moles of nitrate in sample.
- Ion exchange resin in column
- In a small clean beaker, mix roughly 50% of resin and 50% of 1 M NaCl (V/V). (1 M NaCl: 14.61g to 250 ml DI water; Resin: BIO-RAD, AG 1-X8, 200-400 mesh, chloride form, density is 0.75 g/ml).
- Shake the beaker vigorously to homogenize the resin solution.
- Prepare the resin column (SUPELCO, 3 ml empty Fritted SPE tubes) by pipetting 0.6 ml of the above resin solution and transferring to the tube, verify that you have effectively ~0.3 ml of resin. Put a frit on the top of the resin bed in the tube.
- Rinse the resin with 3 x 3 ml of NaCl (1 M) and following with 3 x 3 ml of DI water (using syringe).
- Trap and collect nitrate
- Prepare clean 1L bags, transfer melt water into the bags, connect the bags and packed columns with tubes, and pass the melt water samples through the resin. After the melt water has gone through, rinse the bag with enough DI water (10~15 ml), let the rinse water pass through the tube and column, and drain to a sink.
- Place a small clean container (15 ml dry pre-cleaned bottle) below the column. Label the bottles with sample name, including core number, tube number and sample number.
- Elute the column with 5 x 2 ml 1 M NaCl (using a syringe to push the solution through), collect the solution in the bottle; put caps on and seal the bottles. Freeze bottled samples.
- Rinse the resin with 3 ml of DI water (using syringe). The sample columns are ready to be used for another sample.
- If the resin turns grey or black, or if you suspect any contamination, dispose of column and make new ones. Depending on the purity of the sample, the same resin can be used many times.
HEEL Protocol by Sydney Clark and others
So far I have only linked the original pdf from HEEL, here