CRDS Water Isotope Analysis - δD δ18O - Dr DeSoto
EXPIRED - USE the generalized CRDS procedure.
Introduction
Dr DeSoto is a Picarro L2120i liquid water cavity ring-down spectroscopy (CRDS) laser. dD and d18O of water are derived from absorption of specific spectral lines in the near-infrared range. Water vapor is introduced into an optical measurement cavity with an effective path length of up to 20 kilometers. We have two other Picarro liquid water isotope analyzers, Gorky, a L1102i that also measures dD and d18O and Phoenix, a L2140i that measures dD, d18O, and d17O.
Currently, each injection takes approximately 8 minutes and 30 seconds. A full tray of 54 vials with 10 injections each, then, will take about 3 days and 5 hours.
Safety
Users must be careful with syringe needles and vials. If either of these items are no longer useful (i.e. broken), they must be discarded into the broken glass container. The vaporizer is 110 *C and the septum nut can be hot to touch.
Terse Procedure
- Clean up previous run – close the Coordinator software, remove existing vials.
- Load new waters - load 200 uL of water into 300 uL fused insert vials.
- Take notes - write the tray description and any other notes in the notebook while loading waters into vials.
- Prepare autosampler - put the vials in the tray, clean the syringe, modify the job, don’t change the septum yet.
- Fill out the daily log which is bookmarked on the DeSoto computer.
- Start Run – open Coordinator Launcher, select High Precision, Start Autosampler, click Change Septum, wait for first injection to complete.
- Take more notes – write down time and date of run start, what you did with the syringe, septum etc.
- Change Septum – when the Coordinator indicates the first injection is complete, press “Septum Changed” when done.
- File management – make sure a directory has been created on the server for these samples (e.g. S:\data\projects\...).
- Tray Description – create a tray description file in the newly created folder, use a text editor, save as yymmdd_TrayDescription.csv.
- Data - use Matlab and the gorky script to reduce data from DeSoto.
- Cleanup - empty the existing vials, throw the caps in trash, discard water down the sink, put vials in a beaker in the drying oven, clean vials if needed.
Exhaustive Protocol 
Clean up previous run 
Close the Coordinator...be patient, it will close eventually. Remove vials from the previous run and set them aside for later cleanup.

Load new waters 
Load 200 µL of water into 300 µL fused insert vials. Use the pipette, pipette tips, and trays in 303B drawers labeled ‘Laser’. Vials are located on the lab bench above these drawers. Should you wish to load 1 mL of water, old, 2 mL vials without the 300 µL inserts which are located in a drawer labeled ‘Laser – extras’.
Use one full tray of dry clean pipette tips and also have one empty pipette tray available. For each distinct water, use a clean dry pipette tip, discarding the wet tips into the tray that started out empty. In this way, you will have one pipette tip tray with dry clean tips and one tray to receive the wet tips. If the pipette itself becomes wet, either on the inside or outside, ensure it is dry before proceeding. Use wipes or paper towels and / or compressed air to dry it out.
Make sure the caps on the vials are just tight enough but not too tight. If the cap septum is overly concave, it is too tight. Put all standard waters and all sample waters back in the refrigerator when loading is complete. Put the wet pipette tip tray in the drying oven. As you are loading waters, write the water type or sample ID in the notebook.
Take notes
Write the date, your name, sample set id, and the complete tray description as well as any other notes you desire in the notebook (Dr DeSoto) while loading waters into vials.
Prepare autosampler 
Place the tray of samples and standards onto the tray-holder into either the rear or front position. Make certain the tray is properly seated in one of these two positions. Visually check the caps one last time; no cap should be overly concave.



Autosampler job - Modify the autosampler job according to your current number of vials and your preferred number of injections per vial. Press the F4 button on the autosampler control pad (see photo) to navigate to the home screen. From the home screen on the autosampler controller, turn the wheel to highlight the appropriate job and center click. 'First' should be 1, 'Last' should be the last vial in your tray (e.g. 54), 'Increment' should be 1, 'Count' should be the number of injections per vial you wish to have (e.g. 10). To change any of these values, rotate the wheel until the value is highlighted, center click, and turn the wheel to edit the number. Center click again when you are done editing. Press 'Home' (the F4 button) when you are finished editing the job.
Daily Log 
The lab uses a daily log for each instrument or preparation line to allow users a first glance at the readiness of the instrument. By comparing the current state of the instrument to historical states, you are more informed about the instrument and whether or not it is functioning properly and ready to run your samples.
Each daily log is web based and browser accessible. No link is provided here by design. Open the browser on the controlling computer and you should see at least two tabs already open. One tab is this SOP and the other is the daily log. If the browser has more than two tabs open, it may have additional SOPs. Use the bookmark toolbar as needed if tabs have been closed.
Work through each cell of the daily log. If you are uncertain where to find certain information, hover over the column header tip, denoted by a ⓘ symbol.
Make certain to press the 'save to log' button when you are finished entering data.
You are welcome to make notes if you have observed something with or done something to the instrument and would like to document that information. Use the "insert note" link at the top of the daily log to make a note. You may enter notes at any time.
Start Run 
Open the Coordinator Launcher, select High Precision or High Throughput, then select Start on the Autosampler (F4 from Home screen). Assuming you are running only one tray, rotate the wheel until 'Selected' is highlighted and center click. If you have two trays, select 'All' with a center click. Click "Change Septum" (upper right corner of the Coordinator window) and wait for first injection to complete (~ 8 min 30 sec).
Tray Description 
A good time to create the tray description file is while you are waiting for Dr. DeSoto to be ready to have its septum changed. The tray description file is used by the Coordinator software to assign each injection an Identifier which you give to each vial. As a template, use C:\DrDeSoto\TrayDescriptions\_Rear_TrayDescription.csv (or *\_Front_* if you are loading a tray for the front position). Use a text editor to open one of these templates. If you double click on one of these templates, it will open in Notepad++. The header row in these templates is: "Tray","Vial","Identifier 1","Identifier 2". The Tray and Vial columns are already filled in. Type in the water that is in each vial under Identifier 1. You can leave Identifier 2 blank unless you have extra information to be included. Example Identifier 1 entries are "SW", "WW", or "VW", without quotes. Save this file as yymmdd_[SampleSetID_]TrayDescription.csv (where yymmdd is today's date, and [SampleSetID] is optional and the square brackets are not included). The "TrayDescription" part of the filename is essential for some parts of the matlab code.
Import your tray description file by clicking ‘Load Sample Descriptions’ in the Coordinator software and then browse to the tray description you just saved (c:\DrDeSoto\TrayDescriptions\). Note the software expects you to enter two tray descriptions, one for the front tray and one for the rear tray. The first window prompt is for the front tray ("frnt") and the second window prompt is for the rear tray ("rear"). If you have two tray descriptions, great, otherwise, you can cancel the "frnt" window prompt, then select your rear tray description when prompted.
Change Vaporizer Septum 

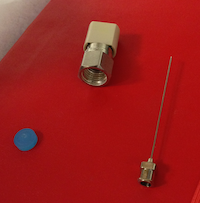
After the first injection is complete, the coordinator will indicate when it's appropriate to change the septum. Remove the magnetic cover and use the slotted wrench side of this cover to loosen the nut (see photo). Remove the nut with your fingers, taking care as it is hot. The septum should be stuck inside the nut. Use the large-gauge needle that is in the syringe cleaning kit to extract the septum (see photo). Discard the old septum into the trash. Place the new septum into Swagelok nut using the blunt end of large-gauge needle. Place the nut back onto the vaporizer, taking care not to allow the septum to fall out when you turn the nut over. Hand tighten the nut; you should feel the nut bottom out. Press “Septum Changed” which is the same button you pushed to begin changing septum in Coordinator window.
Take more notes
Write down the time and date of run start. This is essential as files are named within the instrument based on time. Also write down what you did with the syringe, the septum, etc.
Data 
Check to see if a project directory already exists for these samples on the server. All project directory are located in S:\Data\projects\. It may be that your project is within one of these super-projects. For example, if you are simply running test samples to see if the instrument is working properly, look inside the 'tests' directory. Often times, the directory you are looking for or creating is the sample set ID for the samples you have. Within this directory, create three folders: ‘raw’, ‘reduced’, and 'traydescriptions'. Copy the data from the DeSoto computer located in C:\IsotopeData\ to the 'raw' folder on the server (S:\). Your file will have the date and time you started it as part of the filename (e.g. HBDS2213_IsoWater_20160223_151837.csv) where HBDS2213 is the instrument serial number and the time is so lovingly put in GMT, thanks Picarro (GMT = PDT + 7; GMT = PST + 6). Also copy the TrayDescription file you created on DeSoto to the 'traydescriptions' folder.
Type gorky into matlab command prompt. Browse to raw file you just transferred from DeSoto and open it / select it. Follow prompts in matlab. In the end, a reduced txt file is created
Cleanup
Vials
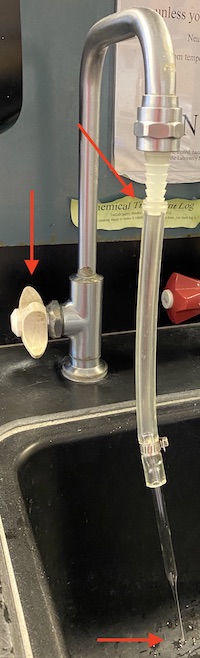
Take vials with previously analyzed water to the wet lab. Open each vial, discard the caps into the trash, and discard the water down the sink by gently shaking the vials. If the vials contained dirty or salty samples, they must be soaked in DI water. You will have to pipette DI water into each vial. You may also use the vial-cleaning-hose-pipette-contraption as shown in image. Once vials are clean, gently place vials in beaker and put them into the drying oven. If soaking in DI water does not get the vial inserts clean, use the glass pipette rubber hose tool attached to a DI water spout to force DI water into the bottom of the insert.
Vaporizer Cleaning
This procedure is completed irregularly depending on the memory or carry-over from sample to sample. More salt buildup inside the vaporizer will leak to more carry-over from previous samples. Picarro wrote up a procedure for this. A pdf of this procedure is located under Resources in Manuals. We have slightly different parts to assemble the apparatus but the general workflow is the same. Also, consider using 5-10% acetic acid as the first rinse of the vaporizer. Follow this by numerous rinses with 18 MΩ water.
Troubleshooting
- The syringe has died - You come in to find the syringe mangled while the autosampler and instrument continue to run. Scroll up in the upper Coordinator window to find where the injections stopped working and note that vial number. Close the Coordinator. Replace the syringe with a new one. Discard dead syringe into the broken glass box located in room 303B under the northernmost sink (since this is not a skin piercing style needle, the broken glass box is sufficient). Modify the autosampler job to start on the vial you noted above. Start the instrument as normal, but don't worry about changing the septum.
- The water background is way too high - Check the valve on the back of the vaporizer to ensure it is pointing towards dry air (see photo at top of this page).
Suggested Reading
- Lis G, Wassenaar LI, Hendry MJ. (2008) High-precision laser spectroscopy D/H and 18O/16O measurements of microliter natural water samples. Analytical Chemistry 80, 287 doi: 10.1021/ac701716q.
- Guidotti S, Jansen HG, Aerts-Bijma AT, Verstappen-Dumoulin BMAA, van Dijk G, Meijer HAJ. (2013) Doubly Labelled Water analysis: Preparation, memory correction, calibration and quality assurance for δ2H and δ18O measurements over four orders of magnitudes. Rapid Communications in Mass Spectrometry 27, 1055. doi: 10.1002/rcm.6540.
- Gupta P, Noone D, Galewsky J, Sweeney C, Vaughn BH. (2009) Demonstration of high-precision continuous measurements of water vapor isotopologues in laboratory and remote field deployments using wavelength-scanned cavity ring-down spectroscopy (WS-CRDS) technology. Rapid Communications in Mass Spectrometry 23, 2534. doi: 10.1002/rcm.4100.
- Wassenaar LI, Kumar B, Douence C, Belachew DL, Aggarwal PK. (2016) Measurement of extremely 2H-enriched water samples by laser spectrometry: application to batch electrolytic concentration of environmental tritium samples. Rapid Communications in Mass Spectrometry 30, 415. doi: 10.1002/rcm.7459.
- West AG, Goldsmith GR, Brooks PD, Dawson TE. (2010) Discrepancies between isotope ratio infrared spectroscopy and isotope ratio mass spectrometry for the stable isotope analysis of plant and soil waters. Rapid Communications in Mass Spectrometry 24, 1948. doi: 10.1002/rcm.5126.
Other Resources
Picarro has a series of tutorial videos that may be helpful.
