Carbonate 17O
Introduction
The carbonate 17O preparation manifold is used to place CO2 and O2 in a confined space with hot platinum to allow for oxygen equilibration among the two species. The O2 source is a compressed gas cylinder and has a known oxygen isotope composition. The CO2 source is your sample or a standard and also, should have a known isotope composition. The most exhaustive measurement strategy is to measure both the O2 and CO2 initial (before equilibration) and O2 and CO2 final (after equilibration. CO2 samples often take one to several hours to prepare. The equilibration process itself takes several hours per sample. As such, any mistake resulting in loss of the sample squanders many hours of your time. Please be careful, methodical, and thoughtful as you move through each step of the below procedure.
The below method uses shorthand like G = gauge, V = valve, LN2 = liquid nitrogen. The octopus is the name for the 8 cold-finger manifold used to collect O2 samples and transfer them to the Lorax mass spectrometer. Recognize that two of these manifolds exists; one is labeled as octopus while the other is labeled as squid. In this document, only octopus is referred to but all instructions could also apply to the squid.
Safety
Appropriate precautions should be taken to protect yourself against the cold and hot temperatures associated with this method. Wear safety glasses and leather gloves when handling liquid nitrogen. Wear leather gloves when handling the heating devices.
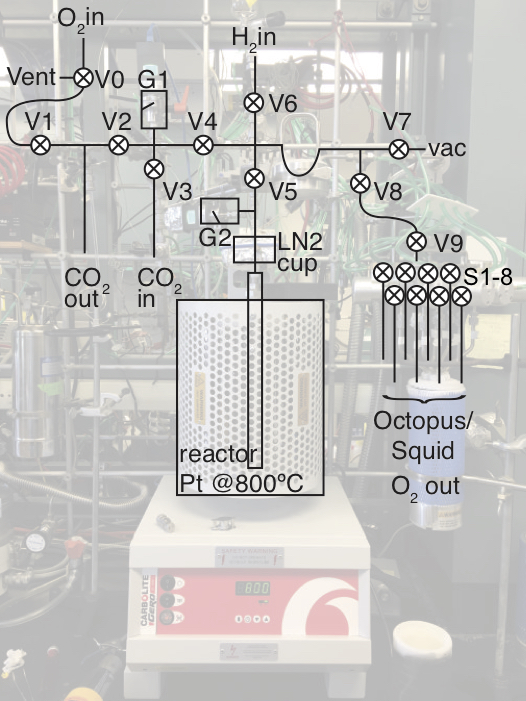
Terse procedure
See Exhaustive Protocol below for more thorough descriptions
- Fill in sample log (sample names, G1 and G2 pressure, etc.)
- Turn off O2 manifold bake-out (turn dial to off, close S1 through S8 on octopus)
- Get set-up: If a sample CO2 tube hasn’t been pumping overnight at CO2-in, attach one and pump.
- Prepare for CO2: Allow pressure to return to background, freeze cup, record G2.
- Introduce CO2: Crack tube to introduce CO2, record G2 pressure.
- Introduce O2: Bleed in O2 to appropriate G1 pressure (set by CO2)
- Start the equilibration: Close V5 and thaw LN2 cup. Record thawed pressure, and let equilibration proceed for at least 60 minutes. Go to step 8 while you wait.
- Collect buddy O2: Freeze octopus and collect O2 in the volume above the reactor.
- Put an empty tube at CO2-out, and pump.
- End the equilibration: Note final pressure, freeze LN2 cup and wait for G2 to reach the value in step 5.
- Prepare for O2 transfer: Freeze trap, check LN2 of octopus
- Transfer O2: Open V5 and transfer O2 until G2 ~<2E-2 Torr. Top off LN2 every ~2 minutes.
- Transfer CO2: Thaw trap and cup and freeze tube at CO2-out. Flame seal tube.
- If you’re running another sample, load it. Consider the next step or return to step 3.
- (Optional) Clean Pt: Add ~100 Torr of O2 to the reactor. Wait 10 minutes, and pump it all away. Add ~100 Torr of H2 to the reactor, and wait another 10 minutes.
- To end the day, usually add a new sample in the cracker at CO2-in. Swap the octopus with one connected to Lorax, and make sure it is baked out.
Exhaustive Protocol
If you’re a pro, see Terse procedure above.
START SAMPLE LOG
- Date: Today’s date as YYMMDD
- Sample prep order: First, second, third, etc. sample of the day
- sample name: the name of the CO2 sample, such as ETH2
- sample detail: the specific digestion of the sample from the polly prep line, such as 190801_1_ETH2
- sample ID: the ID for the sample you’re measuring, which is an equation that combines points a, b, and c above.
- Record G1 and G2 pressures
GET SET UP
At the start of the day, all valves except V6 will usually be open, and V0 should be pointing to the left. A CO2 sample in a sealed tube will typically be pumping in the tube cracker at the CO2-in position, and the melted end of a pyrex tube will be plugging the CO2-out position.
- If a sample CO2 tube hasn’t been pumping overnight at CO2-in, find your sample, score it, and place it in a freshly greased tube cracker.
- Close V3. Attach the cracker at CO2-in.
- Close V2, V5, V6, and V8. Open V3, V4, and V7 to evacuate cracker. A valve is open when the black handle is in line with the valve body, A valve is closed when the handle is at 90º with the valve body.
- Allow G1 pressures to return to <5E-3 Torr, then open V5 and wait for G2 to return to <5E-3 Torr
- Make sure the octopus heater is off and all S valves are closed (on the computer).
- Turn off heater using dial that is beneath the octopus and lower the foil wrapped ring
- Use computer to close S1 through S8.
- If the octopus has not been heating overnight, check the daily log and/or the octopus to see if there is a note that says there are still samples in the octopus from the previous day.
- If this is not your first sample of the day and you installed and pumped out a new CO2 tube during the last equilibration, proceed to step 4.
PREPARE FOR CO2
- Close V5, Add liquid nitrogen (LN2) to the cup, and wait for the LN2 to stop bubbling.
- Top off LN2 and enter the frozen G2 pressure in the daily log.
INTRODUCE CO2
- Close V2, V4, V7, and V8.
- Crack the CO2 tube, wait 30 seconds, and record the pressure on G1.
- Top off cup with LN2
- Open V4 and V5. This will transfer your CO2 sample to the LN2 cup.
- Wait for G2 to reach a stable minimum. This will usually be <5E-3 Torr and take ~2 minutes. Record G2 value in sample log.
- Top off the LN2 cup and open V7 to pump away any residual >5e-3 Torr. Move to the next step if G2 is already < 5e-3 Torr.
- Close V5 to isolate your CO2 sample in the reactor. Close V3.
INTRODUCE O2
When V0 is pointed to the left, a small steady stream of O2 is bleeding into the lab, and the goal here is to switch that flow into the line and get the same amount of O2 into the reactor as there is CO2 from the sample in step 5.
- Take note of the target O2 pressure calculated by the sample log
- Open V1, V2, V4, V7, V8 and V9. All other valves should be closed.
- When G1 is <5E-3 Torr, close V8.
- Turn V0 to the right to allow O2 to enter the line, and wait about 15 seconds for G1 to reach steady state (usually about 9 Torr).
- Top off the LN2 cup. And note your target pressure again. The next step can go quickly so read it before taking action.
- Simultaneously close V7 and open V5. This will divert O2 into the reactor and you will see G2 increase. When G2 approaches the target pressure, simultaneously close V1 and V5. At this point you have an aliquot of O2 in the reactor, and another trapped between V1 and V7.
- Record G2 pressure as “O2 initial frozen G2”
- Turn V0 back to the left
START THE EQUILIBRATION
- Put on eye protection and use compressed air (red tube hanging up and to the left of the line) to blast the LN2 out of the cup. Stop when there is no more liquid in the cup, and don’t use the air to warm the cup.
- It will take ~15 minutes for the cup to warm up, so move on to the “Collect O2 initial” step while you wait.
- After the cup has come to room temperature, record the time and the total pressure in the reactor as “Equilibration Start Pressure” in sample log.
- The goal is for this pressure to be 120 Torr. The larger the difference from this target, the larger the correction.
COLLECT O2 INITIAL
The aliquot of O2 between V1 and V8 that isn’t equilibrated is often called the buddy O2 and is our initial O2 measurement
- Put LN2 under the cold fingers of the octopus. The O2 will be frozen on molecular sieve at the bottom of the cold fingers so the dewar does not need to be filled all the way to the top, but should cover at least 2 inches of the cold finger.
- Note which cold finger will get the buddy. If it’s your first sample of the day, it will usually be #1, if it’s your second sample, it will usually be #3.
- The values on the octopus are pneumatic and are controlled by the computer on the other side of the line (toward the fume hoods). Walk around and click the dark green circle next to the appropriate valve number. It will turn bright green, signalling that it is open.
- Back at the carb17O prepline, open V8, and make sure V9 is open. This will start the O2 transfer, as indicated by a decrease in G1. This transfer takes ~10 minutes, at which point G1 should be <4E-2.
- Walk back to the computer and click on the bright green valve icon. It will turn dark green indicating it is closed.
- Record the residual value on G1 in the sample log.
- Open V7 to pump away any residual gas until G1 ~<3E-3 Torr. Close V8.
- Make sure V0 is pointing left, and then open V1. There is still a lot of O2 between V0 and V1 and this will pump it away
INSTALL EMPTY TUBE AT CO2-OUT AND NEXT SAMPLE IN TUBE CRACKER AND PUMP
- Close V1, V2, and V3.
- Install a new, empty pyrex tube at CO2-out and open V2, V4, and V7 to pump it out.
- Remove tube cracker, remove previous CO2-in tube from tube cracker, clean and regrease as needed, score next sample, install top half of tube cracker below V3, install tube into bottom half of tube cracker, install bottom half of tube cracker onto top half of tube cracker and clamp on, open V3
- When G1 ~<1E-2, open V8
- Allow the tube to pump for the remainder of the equilibration time.
END THE EQUILIBRATION
After equilibrating for an hour, you now need to separate the O2 from the CO2. The general goal of the next few steps is to freeze the CO2, so that the O2 can be moved to a cold finger on the octopus, then thaw the CO2 so that it can be moved to a separate tube.
- Note the final G2 pressure and time.
- Freeze LN2 cup and wait for G2 to reach the “O2 initial frozen” value (from step 6). This can be slow, and take 15 minutes. Be patient, and top off the LN2 every ~5 minutes.
PREPARE FOR O2 TRANSFER
- When G2 has reached the “O2 initial frozen” value (from step 6), record the exact value put LN2 on the U-trap by raising and filling the dewar below it.
- Check that LN2 in the dewar below the octopus is covering at least 2 inches of the cold fingers.
- Close V1, V3, V4, and V7. V8 and V9 should be open (this provides a path for the O2 to reach the octopus).
- Walk around to the computer again and click on the dark green icon for the appropriate value to open it. If this is your first sample of the day, it’s probably #2. If it’s your second, it’s probably #4.
TRANSFER O2
- Top off cup and U-trap with LN2
- Slowly open V5, which will start the transfer of O2.
- Transfer O2 until G2 ~<2E-2 Torr. This will take ~20 minutes, and during that time, you want to make sure you don’t lose any CO2, so it’s important to top off the LN2 cup and U-trap every ~2 minutes.
- When G2 ~<2E-2 Torr or you’ve waited much longer than 20 minutes, walk back around to the computer and click the bright green icon to close the valve.
- Record the O2 transfer residual G2 value in the log file
- Top off LN2 cup and U-trap, then open V7 to pump away any residual gas until G2 <~3E-3 Torr.
- Close V7, V8, and V9.
TRANSFER CO2
- Put a dewar with LN2 under the tube attached at CO2-out. Use the ring stand, ring, and square of plywood to support the dewar, then add LN2 to cover the bottom ~1 inch of the tube.
- Open V2, V4, and V5, which will allow the CO2 in the LN2 cup, and any in the U trap, to move to the tube. If G1 shows a large increase in pressure during this step, you probably didn’t pump out the tube sufficiently, and this should be noted.
- Put on eye protection and use compressed air to blast the LN2 out of the cup, and U-trap. Stop when there is no more liquid in the cup, and don’t use the air to warm the cup.
- It will take ~15 minutes or more for the LN2 cup and U trap to thaw, and as they do, their CO2 will be transferred to the tube. You can lower the dewar on the U-trap to speed up the thaw process. Later, you can help thaw the U-trap and cup by putting your warm fingers around them, but avoid using the heat gun. Top off the dewar with LN2 every ~5 minutes so that the bottom of the tube stays frozen.
- When the cup and U trap are close to room temperature, and G1 is ~<3E-3 Torr and stable, top off the dewar at CO2-out with LN2 and record the residual G1 value in the log.
- If G1 does not drop below 5e-3 Torr and it is stable, record the residual G1 value in the log and open V7 to pump away the residual gas.
- Close V2, and flame seal the tube. See the Polly Prepline Method for how to successfully flame seal. The torch is shared with the Polly Prepline, and will likely have to be passed awkwardly under Polly Prepline and across the aisle to the carb17O line. If you are having trouble lighting the torch, check that the torch O2 cylinder (to the right of fume hoods), and gas valve (labelled “clump” between fume hoods) are open.
- Let sealed tube remain in LN2 until it’s tip is cool enough to touch. Wipe it off, and clearly label it with a tape flag that provides its sample ID followed by “CO2_f”. Place tube in the drawer until it’s time to run on Polly.
PREPARE FOR ANOTHER SAMPLE
If you are going to equilibrate another sample, you need to make sure the line is pumped out and ready.
- Close V2, V5, and V8, and make sure V7 is open.
- Open V3 and watch G1. It will probably increase a bit but should drop to below 5e-3 Torr. If it does not, rotate the tube cracker greased joint and / or tighten top and bottom ultra-torr fittings.
- Once G1 is below 5e-3 Torr, open V2 and V1. Again, wait until G1 is below 5e-3 Torr.
- Open V8 and V9 and wait for G1 to be below 5e-3 Torr
- Open V5 and wait for G1 to be below 5e-3 Torr
- Now start back at the top of this procedure. Consider the following optional step before doing so.
Clean Platinum Catalyst (Optional)
There’s some evidence from before Andy was born that any CO that remains on the platinum in the reactor can be “cleaned” with a treatment of pure O2 (to convert CO to CO2), followed by a treatment of pure H2 to convert any residual O2 to water. We currently don’t know if it’s useful, so are trying to do it about half the time to evaluate if there is a difference. Whether you do this step or not, note it in the daily log. It seems most convenient to complete this step at the end of the day.
- Make sure the prepline fully pumped out, and all valves are open except the octopus valves controlled by the computer.
- Close V3, V5, V6, and V8.
- Turn V0 to the right and wait ~10 seconds for G1 to stabilize.
- Close V7, and open V5 to add O2 to the reactor until G2 is at ~100 Torr.
- Close V5, and turn V0 back to the left
- Let the O2 sit in the reactor for ~10 minutes. While you wait, open V7 to pump away the extra O2.
- After 10 minutes, open V5 and pump until G2 <1E-2 Torr. Mixing oxygen and hydrogen at 800 ºC will combust, so it is critical to get all the oxygen out before adding hydrogen!
- Pump out flask neck by closing V2, V3, V5, and V8. Open V4, V6, and V7 and wait for G1 to drop below 5e-2 Torr. Close V4, V6, and V7.
- The glass flask above V6 has hydrogen in it, but exactly how much will vary with time. Your goal is to get ~100 Torr of H2 to the reactor, but exactly how is a bit of a guessing game.
- With V6 closed, start by opening the lower black screw valve on the glass flask, wait a moment, and then close it again.
- Open V6, then V5, and note the pressure on G2
- If G2 pressure is ~50 Torr or more, close V5 and repeat step k until G2 is in the 80-120 Torr range.
- If G2 pressure is <50 Torr, close V5, but leave V6 open. With V4, V7, and V8 closed, open the lower black screw valve on the glass flask, wait a moment, and close it again. Open V5 and note the pressure. Repeat until G2 is in the 80-120 Torr range.
- If G2 pressure is <10 Torr, you need to add hydrogen to the glass flask. See instructions below.
- Once G2 is in the 80-120 Torr range, close V5, and wait ~10 minutes
- While you wait, open V7 to pump away extra hydrogen.
- After 10 minutes, close V6 and open V5 and V7 to pump away all hydrogen
- Open all other valves except V6 to pump until you’re ready for the next sample.
START MEASURING O2 IN OCTOPUS ON LORAX
- Make sure V8, V9, and all the octopus values are closed.
- Drop the LN2 dewar from below the octopus cold fingers
- Disconnect the black valves that connect the green air hose to each octopus valve. These are a little tricky, but push the tube in while compressing the ring on one end of the valve, and you will be able to pull it out.
- Loosen the Swagelok ultra-torr fitting, and carefully pull the octopus away from the prepline.
- Walk the octopus into the mass spec lab and set it down in a stable place near Lorax (e.g. the black bench top in front of the computer). Rest it on its side so that the cold fingers are still angled downward (NEVER TURN THESE MANIFOLDS UPSIDE DOWN, the molecular sieve could become lodged higher in the cold finger making that cold finger useless).
- Prepare Lorax for octopus removal
- Make sure the previous run has finished.
- In IsoDat on the Lorax computer, close all 8 octopus valves (S1 through S8)
- close the valve leading to the external port (Dual Inlet System@Valve 11).
- Open Dual Inlet System@Valve 39 (which will close Dual Inlet System@Valve 40)
- Close V9 for the octopus attached to Lorax, and disconnect the valves from the air hoses in the same way as above.
- Loosen the Swagelok ultra-torr to pull the octopus away from Lorax. Set it aside in a stable place.
- Attach the octopus with the day’s equilibrated oxygens at the Swagelok ultra-torr fitting, and open “Dual Inlet System@Valve 11” in IsoDat.
- While you wait for pressure to return to background (<2e-3 mbar on gauge located lower center of dual-inlet window), connect the valve hoses and double check that they are tightly connected.
- Open V9 on the newly attached octopus after dual-inlet gauge has reached minimum.
- Close previous *__Finish_Run-0000.dld window
- If “carb17O.seq” is not already open, find it in the sequences tab of the File Browser window on the left side of Isodat Acquisition.
- Prepare the sequence for today’s oxygens
- Each O2 sample is multiple rows associated with it. All samples have two “conditioners” and the first sample of the manifold has three “conditioners”. These are analyses that will be discarded as part of the data reduction. The O2-initial samples have three rows while the O2-final samples have 10 rows.
- Type the sample ID for each O2-initial (O2in) and each O2-final (O2f) under the Identifier 1 column heading.
- Identifier 2 is the octopus cold finger number.
- The very first row of a sample (Identifier 1 == conditioner) should have all four checkboxes checked and a method “O2_Octopus_cndtnr.met”.
- The remaining rows of each sample should have the checkboxes “Peak Center” and “Pressure Adjust” checked and a method “Water_17Oxs.met”.
- Daily Log
- The lab uses a daily log for each instrument or preparation line to allow users a first glance at the readiness of the instrument. By comparing the current state of the instrument to historical states, you are more informed about the instrument and whether or not it is functioning properly and ready to run your samples.
- Each daily log is web based and browser accessible. No link is provided here by design. Open the browser on the controlling computer and you should see at least two tabs already open. One tab is this SOP and the other is the daily log. If the browser has more than two tabs open, it may have additional SOPs. Use the bookmark toolbar as needed if tabs have been closed.
- Work through each cell of the daily log. If you are uncertain where to find certain information, hover over the column header tip, denoted by a ⓘ symbol.
- Make certain to press the 'save to log' button when you are finished entering data.
- You are welcome to make notes if you have observed something with or done something to the instrument and would like to document that information. Use the "insert note" link at the top of the daily log to make a note. You may enter notes at any time.
- Highlight the rows to be analyzed in the sequence by clicking and dragging down with the pointer in the “Row” column.
- Scroll down to the base of the sequence, hold down the “Ctrl” key on the keyboard, and click the very last row called “Finish_Run”.
- Click Start
- Leave all fields as is within the window that popped up
- Click OK and you are done
- Each equilibrated sample (O2in and O2f) takes about 10.5 to 11 hours.
PREPARE carb17O PREPLINE FOR OVERNIGHT
- If you are re-attaching the octopus
- Take the empty octopus that you just removed from Lorax and attach it to the carb17O line with the Swagelok ultra-torr fitting.
- Connect all the air hoses, double check that they are tightly connected.
- Close V2, V3, V5, V6, and V8. V4 and V7 should be open.
- Open V8 and wait for G1 to be ~<1E-2 Torr.
- While you wait, put the foil wrapped ring around the bottom of the cold fingers and turn on the heater to max.
- Open V9, and all eight octopus valves (on the computer).
- Leave the octopus baking for at least 10 minutes. This will heat up the molecular sieve, releasing any residual gas. If you are waiting at the line, watch for G1 to rise, and fall back to background.
- Do not leave the octopus valves open without the heat on.
- If you have turned the heater off, close all octopus valves using the computer. Leave V9 open.
- If you are leaving the octopus overnight with O2 samples in it
- All of the octopus valves should be closed, close V8 and V9.
- Drop the LN2 dewar.
- Do not heat the cold fingers overnight.
- Leave a note in the daily log which fingers have samples.
- Make sure V0 is pointed to the left.
- Open all other valves except V6 (and V8 and V9 if you did step b) to pump overnight.
- Close the torch O2 and gas valves.
- Pour LN2 back into large dewars.
RUN CO2f SAMPLES ON POLLY
- Refer to Polly analysis method to help fill in the gaps of the below procedure.
- You may accumulate CO2f samples until Polly is available. Try to assess Polly’s schedule and fit in your CO2f samples. You can also run CO2f and polly clumped samples in a single sequence, but recognize that they run different methods.
- If you are running carb17O CO2f samples without any clumped isotope samples, use the sequence called “17O_CarbLine_Cracker.seq”. If you are running both carb17O and clumped isotope samples, use “17O_CarbLine_Cracker_PollyPrep.seq”. Refer to “Polly Sample Analysis” method for specifics on analyzing a clumped isotope sample.
- Within a sequence, carb17O CO2f samples should always use method “Cracker_17O_CarbLine.met”.
- Type the sample ID (yymmdd_n_id_CO2f) under Identifier 1. These samples only occupy one row in the sequence (unlike the oxygen samples on lorax or the clumped samples here on polly).
- All checkboxes should be checked for the carb17O samples and make sure the Multiport Inlet is appropriately numbered.
- Leave the rows containing zeros alone. Polly runs working reference gas against itself (zero enrichment) when no samples are being analyzed.
- Daily Log - fill out the daily log (see Polly Sample Analysis)
- Highlight the rows to be analyzed in the sequence by clicking and dragging down with the pointer in the “Row” column. Assuming you do not have 10 CO2f tubes to analyzed, let go of the mouse button when you reach the last CO2f row in the sequence, hold down the “Ctrl” button on the keyboard, and then click and drag to the bottom of the sequence to highlight all of the zeros. This helps ensure zeros will run until the next user is ready to load samples.
DATA
Data processing can be done immediately after Lorax and Polly runs are complete, or as a batch anytime afterward.
- Move O2 files to the S drive
- From the “Dual Inlet Results” shortcut on the desktop of the Lorax computer, you should see all your runs (and possibly those of others) organized by date.
- In a separate window navigate to S:\Data\projects\Saenger\Carb_17Oexcess\octopus_raw_files\archive. The most recent O2 file is the last run to be processed, so you will start with the next most recent run.
- For each run not already in the archive folder, open the folder, then open the Excel folder, and copy the .csv file into S:\Data\projects\Saenger\Carb_17Oexcess\octopus_raw_files\
- Move CO2 files to the S drive
- From the “Results” shortcut on the desktop of the Polly computer, you should see all your runs (and those of others) organized by date.
- In a separate window navigate to S:\Data\projects\Saenger\Carb_17Oexcess\octopus_raw_files\archive. The most recent CO2 file is the last run to be processed, so you will start with the next most recent run.
- For each run not already in the archive folder, open the folder, then open the Excel folder, and copy the .csv file into S:\Data\projects\Saenger\Carb_17Oexcess\octopus_raw_files\
- Open each CO2f Excel file and delete any polly clumped data or zero data from the CO2f file.
- Split up files
- Move to a separate data processing computer (e.g. North Going Zax or South Going Zax)
- Open MatLab, and for each of your runs, run carb17O_splitO2Files.m and carb17O_splitCO2Files.m, which will separate out each O2in, O2f, CO2f into individual files.
- Copy O2in sample files to S:\Data\projects\Saenger\Carb_17Oexcess\O2_Cylinder\raw
- Copy O2f and CO2f files to S:\Data\projects\Saenger\Carb_17Oexcess\Equilibrations\
- Move the original .csv file for each CO2 and O2 run to the archive folder
- Reduce O2 data
- In MatLab, run carb17O_O2Cyl.m for each new O2in file
- This script does background corrections, and converts mV data to delta notation relative to VSMOW, and writes this information to the log file (Saenger_O2_Cylinder_log.txt)
- Put it all together
- Get prepared by opening
- Saenger_O2_Cylinder_log.txt file (located in S:\Data\projects\Saenger\Carb_17Oexcess\O2_Cylinder\)
- Polly_CO2in.xlsx file (located S:\Data\projects\Saenger\Carb_17Oexcess\)
- gsheet carb17O_PrepLine_SampleLog (https://docs.google.com/spreadsheets/d/11IcIHuQpKlFPJsN63V5CybBrEbGZ33x9Hul3obH81fM/edit?ts=5cd5ad76#gid=0)
- In MatLab, run carb17O.m
- This script calculates d17O of CO2 in and ∆17O of CO2 following either Mahata et al. 2013 or Barkan and Luz 2015.
- You will be asked to provide a number between 1-5 that specifies the sample type:
1 = CO2-H2O equilibration for establishing the empirical fractionation factor (e.g. FC_WW). This DOES NOT include CO2-H2O for clumped isotope reference frame.
2 = cylinder CO2 (e.g. FF or FC)
3 = polly reference frame gas (e.g. FF_SP_23)
4 = carbonate standard (e.g. ETH 1-4 or C64)
5 = carbonate sample - Enter the d13C and d18O of initial CO2 using the Polly_CO2in file. Use d18O VSMOW values for CO2in.
- Enter the d18O of initial O2 using Saenger_O2_Cylinder_log.txt
- Enter equilibration time from carb17O_PrepLine_SampleLog
- Enter O2 initial pressure (frozen G2) from carb17O_PrepLine_SampleLog
- Enter equilibration start pressure
- Leave mass spec pressures blank if you don’t know them
- Enter any comments
- All data goes to the Equilibrations_Log.txt file (located S:\Data\projects\Saenger\Carb_17Oexcess\)
- Run carb17Ob.m
- This script screens out “bad” data and accounts for the empirical fractionation factor using VW and SW CO2-H2O equilibrations. WW is reserved to evaluate accuracy CO2in.D17O_Hofmann_calc values are written to Equilibrations_Log.txt and this can be considered your final data.
- You can also run carb17O_O2Cylb.m to view all O2in data.
Good job!
Suggested Reading
- Barkan E, Luz B. (2012). High-precision measurements of 17O/16O and 18O/16O ratios in CO2. Rapid Communications in Mass Spectrometry 26. doi:10.1002/rcm.6400.
- Mahata S, Bhattacharya SK, Wang C-H, Liang M-C. (2013). Oxygen Isotope Exchange between O2 and CO2 over Hot Platinum: An Innovative Technique for Measuring Δ17O in CO2. Analytical Chemistry 85. doi: 10.1021/ac4011777.
- Passey BH, Hu H, Ji H, Montanari S, Li S, Henkes GA, Levin NE. (2014). Triple oxygen isotopes in biogenic and sedimentary carbonates. Geochimica et Cosmochimica Acta 141. doi: 10.1016/j.gca.2014.06.006.
- Wostbrock JAG, Cano EJ, Sharp ZD. (2020). An internally consistent triple oxygen isotope calibration of standards for silicates, carbonates and air relative to VSMOW2 and SLAP2. Chemical Geology 533. doi: 10.1016/j.chemgeo.2019.119432.
